Recent Posts
- Swing Against Cancer 2025
- New grant funds first-of-its-kind gene therapy to treat aggressive brain cancer
- Study links PFAS contamination of drinking water to a range of rare cancers
- USC study explores new insights into innate resistance for immunotherapies in colorectal cancer
- An early blood test can predict survival in patients with metastatic prostate cancer, shows USC study
New Study Demonstrates Feasibility of Non-invasive Profiling of Advanced Prostate Cancer by Combining Liquid Biopsies with Radiomic Analysis of CT Scans
By Hinde Kast
May 9, 2022

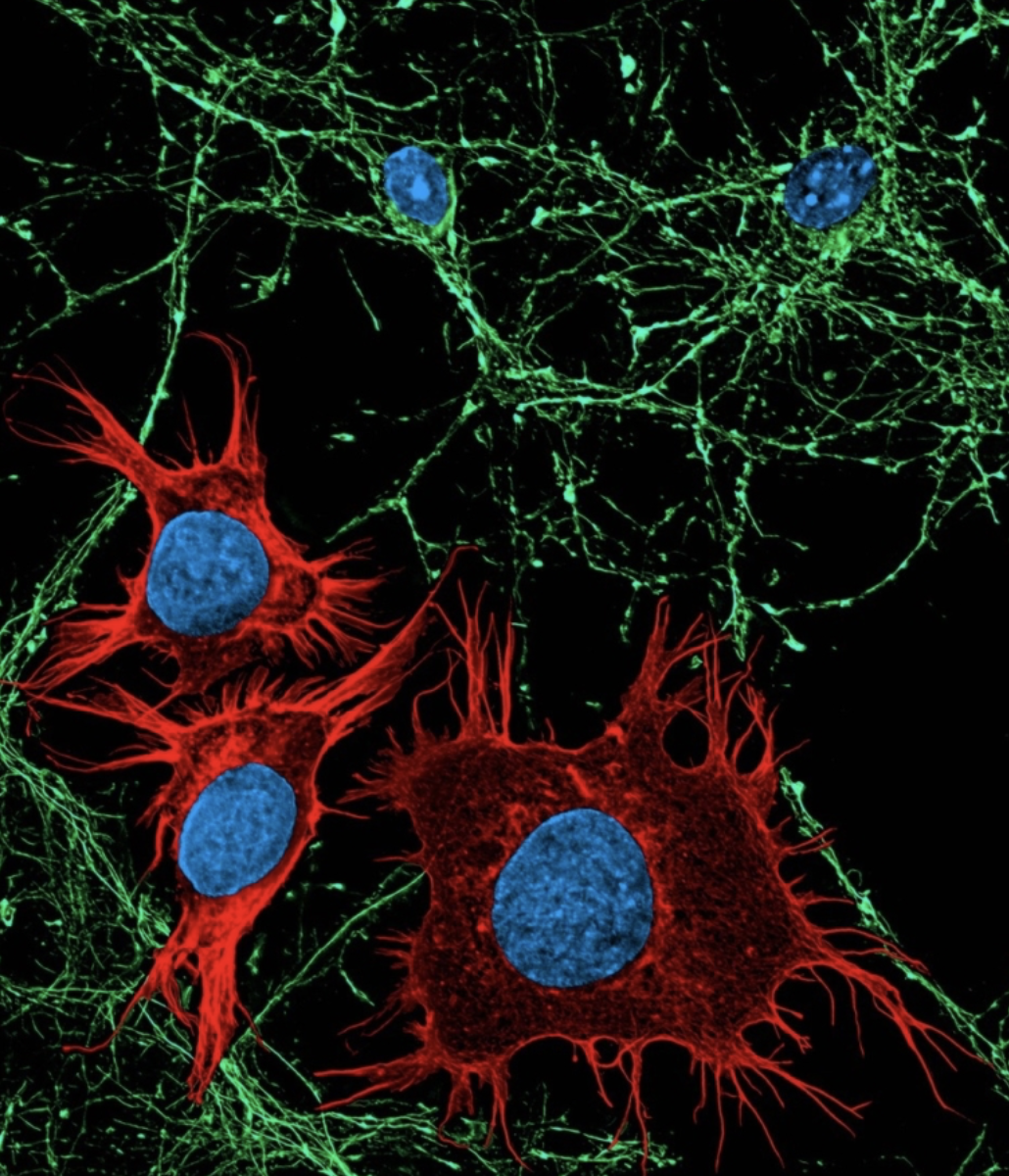
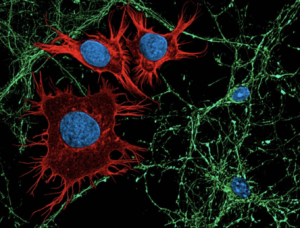
Cancer can evolve and adapt during therapy, leading to resistance and progression. New non-invasive technologies can track these changes in real-time, but to date, these approaches have been developed in isolation, which only allows physicians to obtain a partial idea of what is happening with a patient’s tumor.
Now, a new study published in the International Journal of Molecular Sciences has demonstrated the feasibility of using a single tube of blood and computerized tomography (CT) scans to construct a complex cellular, molecular, and image profile of metastatic prostate cancer in real-time. Such a comprehensive cancer profile may help clinicians predict treatment response, disease progression and overall survival, leading to the development of better treatments.
“Having a more comprehensive picture of tumor biology provides a better understanding of disease progression and resistance mechanisms, which can lead to better therapies,” says study author Dr. Amir Goldkorn, Associate Director for Translational Research at the USC Norris Comprehensive Cancer Center. “Having developed these capabilities, we are now excited to clinically validate the feasibility of this new approach in a large, national phase III trial for men with newly diagnosed metastatic prostate cancer, where these techniques will help us to track cancer progression and predict clinical outcomes.”
The two non-invasive technologies used in this study were liquid biopsy and radiomic analysis. Liquid biopsy detects rare material shed by tumors into the bloodstream, known as circulating tumor cells (CTCs) and cell-free DNA (cfDNA). Radiomic analysis uses artificial intelligence (AI) algorithms to analyze image features in a CT scan. For this study, Dr. Goldkorn and his colleagues at USC Norris, Drs. Vinay Duddalwar and Tim Triche, combined cellular and molecular analysis of CTCs and cfDNA in blood samples with radiomic analysis of CT scans from 22 men with metastatic prostate cancer. They found that the radiomic CT scan analysis correlated with the liquid biopsy analysis. For example, some CT scan features were correlated with higher numbers of CTCs and DNA in the blood. Integration of data from the two complementary tests could provide a more comprehensive, non-invasive way to profile cancer and predict how the disease will respond to treatment.
The research team is now further testing the predictive value of this combined non-invasive approach to cancer profiling by correlating the results with clinical outcomes such as disease progression and overall survival in a multi-center phase III clinical trial for patients with advanced prostate cancer. Their studies are funded by a newly-awarded NCI R01 grant.
About this study:
In addition to Drs. Amir Goldkorn, Vinay Duddalwar, and Tim Triche, other contributors from the USC Norris Comprehensive Cancer Center and the Keck School of Medicine include Drs. Jonathan Buckley, Steven Cen, David Quinn, Yi-Tsung “John” Lu, and Bino Varghese. Other major contributions were made by Children’s Hospital Los Angeles, Thermo Fisher Scientific, and RareCyte, Inc.
The study was funded in part by the National Institutes of Health (1R01CA257610-01) and the USC Norris Comprehensive Cancer Center (P30CA014089). Drs. Goldkorn and Triche have received in-kind reagent contributions from RareCyte and Thermo Fisher Scientific. Dr. Duddalwar received research grants from Samsung Healthcare.








 There are currently more than 3.8 million breast cancer survivors living in the U.S., yet three out of 10 women with invasive breast cancer will develop metastasis in their lifetime, meaning cancer that has spread to other organs. This is a source of worry for both patients and families. However, breast cancers that are not fully cured after treatment are often too small to be detected by mammograms or ultrasounds but pose a significant risk.
There are currently more than 3.8 million breast cancer survivors living in the U.S., yet three out of 10 women with invasive breast cancer will develop metastasis in their lifetime, meaning cancer that has spread to other organs. This is a source of worry for both patients and families. However, breast cancers that are not fully cured after treatment are often too small to be detected by mammograms or ultrasounds but pose a significant risk. 


 The
The 
 To accelerate the development of groundbreaking cancer treatments, the
To accelerate the development of groundbreaking cancer treatments, the